Avéli, a one-time treatment for tackling cellulite on the buttocks and thighs of adult women received another FDA greenlight Thursday, Sept. 1, 2022.
The product is now indicated for long-term reduction of the appearance of cellulite.
Since the cellulite procedure was already in use across the U.S., you’re probably wondering what’s different now and what it means to you.
In short, Thursday’s news stands as yet another vote of confidence for the product.
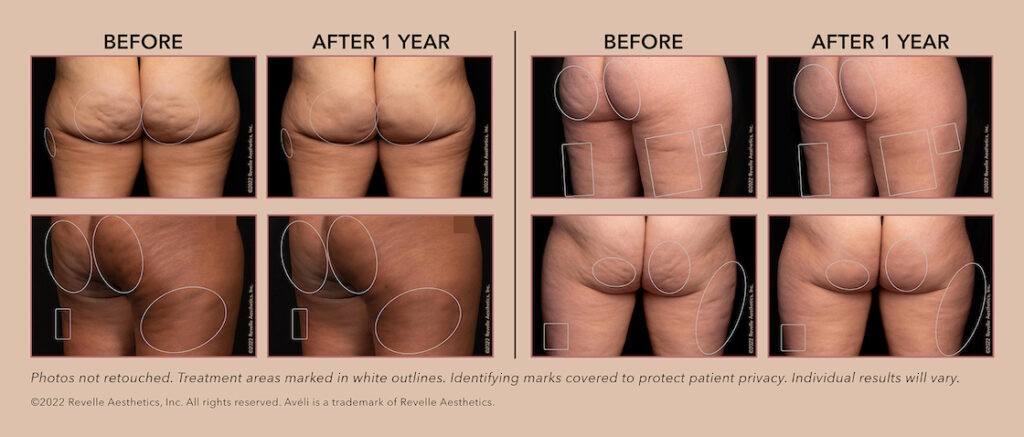
Avéli had previously been studied and shown effective with results lasting three months.
Avéli’s producer, Revelle Aesthetics, Inc., next went on to evaluate its efficacy on a 12-month timeline.
It proved effective, according to clinical data showing that the results of the one-time cellulite treatment last as long as 12 months.
That’s what the FDA’s extended clearance represents.
It’s yet another promising nod to the product that entered the cellulite treatment space in March of this year.
It’s a vertical that’s been plagued by less-than-satisfactory treatments which, while many patients have found success in, dissatisfaction rates have numbered as high as 40 to even 70 percent.
And how does Avéli fare in the bumpy battle?
Thus far, the Avéli treatment’s been given a 100 percent “Worth It” rating on RealSelf.com, but note that it’s only racked up six patient reviews so far.
Avéli is now available nationwide, growing rapidly in plastic surgery and dermatology practices that are focused on the rising needs in body contouring.
“For far too long, millions of women have struggled to feel comfortable in their own skin because of their cellulite and have been settling for less than effective treatment options. Avéli is setting out to change this by finally providing women with a meaningful and lasting solution that targets their cellulite in the areas that have frustrated them the most, their buttocks and thighs,” said Caroline Van Hove, President and CEO of Revelle Aesthetics, in a news release.
“Equally as exciting is the role that we believe Avéli will play as a cornerstone in the latest and fastest growing category in medical aesthetics, lower body rejuvenation.”
Cellulite dimples can be of any shape or size and typically appear on the buttocks and thighs of adult women.
The condition affects up to 90 percent of women, though it produces no negative effect beyond an occasionally undesirable cosmetic appearance.
Fibrous septae are to blame for the appearance of cellulite—although a large number of factors determine whether those fibrous bands end up causing visible cellulite.
These factors include genetic, hormonal, dietary, and lifestyle choices.
Most cellulite treatments target the subdermal fibrous bands in one way or another to render them unable to cause cellulite.
Aveli does so with a precision device used to physically severe the fibrous bands tethering the skin to the muscle below, and thus releasing the divots and dimples in real-time.
“Unlike many current cellulite options, Avéli addresses cellulite from the inside-out,” the company shared upon receiving its extended FDA clearance.
“It is the only minimally invasive procedure that allows a provider to identify which of the fibrous septa bands are causing a cellulite dimple, and then confirm in real-time they are releasing those targeted septa to deliver visibly smoother skin. Results are visible quickly after a single in-office procedure with little-to-no downtime.”


